作者简介:殷志刚(1978-),男,博士,高级工程师,主要从事高能量密度和快充电池的开发和相关机理研究。
提高锂离子电池快速充电能力是当前电池发展的重要方向。快速充电电池应具有优异的锂离子扩散能力,其开发需要从正极和负极角度选择合适材料,同时也要考虑电解液对快充性能的影响。基于现有文献资料,从多维度出发概括总结了开发具有快充性能电池的方法,包括改善电解液的扩散性能,降低去溶剂化势垒和改善固体电解质界面(SEI)膜性能。低熔点溶液、高溶质浓度、高离子迁移数均能改善电解液扩散性能,提高电池的快充性能。选择低溶剂化结合能电解液能够提高电池快充性能。电解液溶剂的选择、溶质浓度的改变、电解液添加剂的加入均能改善负极SEI膜的性质进而提高电池快充性能。电解液开发和创新是快充电池性能提高的关键要素。
Improving the rapid charging capability of lithium-ion batteries is an important development direction of battery. Fast charging battery should have excellent Li+ diffusion ability, and its development needs to select materials in view of positive and negative electrode, and the influence of electrolyte also needs to be considered. Based on the literature, the methods for developing fast charging batteries were summarized from multiple aspects, including improving the diffusion performance of electrolyte, reducing the desolvation barrier and improving the performance of solid electrolyte interface (SEI) film. Low melting point solutions, high solute concentration and high ion migration number can improve the diffusion performance of electrolyte to enhance fast charging performance of battery. Selecting electrolyte with low solvation binding energy can improve the fast charging performance of the battery. The reasonable choice of electrolyte solvent, the change of solute salt concentration and the addition of electrolyte additives can improve the properties of the negative SEI membrane to enhance the fast charging performance of the battery. The development and innovation of electrolyte are key elements to improve the performance of fast charging batteries.
当前, 我国社会经济处于快速发展的关键时期, 对能源的需求量呈现不断上涨的态势。解决能源短缺问题已上升为国家的重大战略, 为此我国政府推出一系列政策来鼓励支持新能源产业的发展[1, 2, 3]。大力发展新能源汽车产业、鼓励安装光伏发电项目、推进开发清洁的风能资源等, 能够有效实现国家能源自给和能源环境的安全。我国一年的石油进口量通常为5亿吨左右, 燃油汽车对石油消耗量占石油需求总量的70%左右, 接近5亿吨, 因此如果新能源汽车取代燃油车将能逐步解决我国石油依赖进口的问题[4, 5, 6]。不少国家宣布将禁售燃油车, 并给出燃油车全面退出的时间表。其中挪威限定间最短, 将于2025年实施禁售传统燃油车; 荷兰将在2030年实施传统燃油车禁售政策; 德国、印度和美国加州政府都把燃油车的禁售年限定到2030年; 法国和英国计划于2040年禁售传统燃油车。我国将会推行2030年商用车实现全电动化, 包括公交车和出租车。2040年, 一线城市燃油车将退市。2050年, 除大型专业车辆外, 新能源汽车市场份额将达到85%[7, 8, 9]。购买新能源汽车的决策因素包括续航里程、充电体验、电池衰减造成的全寿命使用成本、售后体验、舒适度、动力配置等, 是现有用户考虑增换购的主要决定因素。在购买决策因素中, 续航里程和充电体验是重要衡量指标。纯电动汽车用锂离子电池的充电时间普遍在1 h左右, 充满电所需要的时间是普通燃油车加满油所用时间的20倍左右。因此, 电动汽车充电速度已成为消费者最关心的问题之一[10, 11, 12]。若电池的充电时间缩短到10 min以内能解决电池的续驶里程过短和充电时间过长的问题。因此提高电池的充电倍率以缩短电池充电时间成为动力电池发展重要方向[13, 14, 15, 16]。
锂离子电池由正极材料、负极材料、隔膜材料和电解液材料四大核心部分组成, 每种材料对电池的性能均产生重要影响。电池若要实现快充功能需要电解液具有高的锂离子传输能力, 锂离子传输快慢与电解液性能直接相关[17, 18, 19, 20]。如图1(a), 锂离子电池在充电过程中将经历四个过程:(1)锂离子与溶剂分子形成溶剂化锂, 溶剂化锂在电势差和浓度差的驱动下进行液体扩散; (2)在固态电解质界面(sold electrolyte interphase, SEI)膜界面处溶剂化锂离子将与溶剂分子分离即去溶剂化过程; (3)去溶剂化锂离子在SEI膜中传输; (4)锂离子在活性材料本体内传输形成插层化合物[21, 22, 23]。
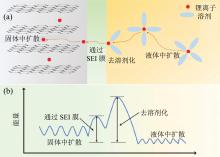 | 图1 (a)锂离子电池充电过程示意图; (b)充电过程不同阶段能量势垒示意图(修改自文献[23])Fig. 1 (a) The charging process diagram of Li-ion battery; (b) the energy barriers diagram of Li-ion battery at different charging process (modified from reference [23]) |
锂离子电池在充电过程中需要克服相应的能量势垒方可实现锂离子的移动, 如图1(b)。其中去溶剂化过程需要的能垒最大, 其次是锂离子在SEI膜中的扩散。在锂离子电池慢速充电过程中能量势垒对电池的影响相对较小, 而当电池需要实现快速充电时, 这些在慢充过程中可忽略的因素将严重影响电池的快充性能。上述四个过程中前三个过程均与电解液的性能直接相关。其中锂离子在电解液中的传输与溶质锂盐及溶剂的性质有密切联系。去溶剂化过程则与溶剂的成分密切相关。锂离子在SEI膜中的传输与SEI的性质有关, 而SEI的性质由电解液溶剂及相关添加剂决定。因此改善电解液的物理、化学性能将有效提高电池的快速充电能力[24, 25]。
锂离子电池快充过程涉及高的锂离子流速, 很容易导致锂枝晶在负极表面的生长和SEI膜的重复形成。这些现象的出现易使得电池发生大的极化、低的充放电库伦效率、电池温度过快升高和安全系数降低[26, 27, 28, 29, 30]。因此开发具有克服上述问题的快充电解液成为必然。
快充电解液的设计开发可从提高锂离子在电解液中扩散速度、降低去溶剂化势垒、优化SEI膜以实现具有高离子导电性等方面开展工作。
当前锂离子电池用电解液溶剂主要包括具有链状和环状结构的碳酸脂类及羧酸酯类, 各溶剂的物理化学性质见表1。
| 表1 不同溶剂物理化学性质[31, 32, 33, 34, 35, 36] Table 1 Physical and chemical properties of different solvents[31, 32, 33, 34, 35, 36] |
有机液体电解液中存在Li+、阴离子和溶剂分子, 这三种物质的自扩散系数关系为Li+ < 阴离子 < 溶剂。虽然Li+半径最小, 然而Li+会与溶剂分子形成溶剂化的Li+而导致具有更大的半径, 这将显著降低Li+的扩散速度[37, 38]。电解液溶剂在常温下熔点越低, 黏度越小, 这对溶质在溶剂中的扩散越有利。因此, 溶质在低熔点溶剂中的扩散速度将越大。KONDOU等[39]以双氟磺酰亚胺锂(Li[FSA])为溶质, 溶解到不同溶剂中构成电解液, 其中电解液的浓度为1 mol/L。不同种类电解液的物性参数见表2。
当电解液的黏度越低时, 离子导电性越强。离子导电性是由阳离子和阴离子的导电性共同决定, 而阳离子、阴离子导电性与相对应的离子自扩散系数存在正比关系。当采用MA作为溶剂时溶液显示出高的锂离子自扩散系数, 同时阴离子和溶剂均呈现最高自扩散系数, 因此该电解液能够提高电池的快充性能。
KONDOU等[39]研究了具有不同溶质浓度的电解液的Li+扩散系数, 发现电解液的浓度直接影响Li+的扩散速度, 如图2所示。横坐标为溶剂浓度与Li+浓度比值, 当数值减小时意味着Li+浓度相对于溶剂浓度增加。纵坐标为溶剂扩散系数Dsol与锂离子扩散系数DLi的比值, 若锂离子扩散系数与溶剂扩散系数同时同比例增加时, Dsol/DLi将保持比例关系不变。若比例关系变大表明锂离子扩散系数改变不如溶剂扩散系数的改变, 反之若比例关系变小表明锂离子扩散系数的改变要明显强于溶剂扩散系数的改变大。Li[FSA]在溶液中会以正电荷Li+和负电荷FSA- 存在, 随Li[FSA]浓度的增加正负电荷的扩散系数均增加, DFSA/DLi呈水平直线。而有时正负电荷扩散系数的增加程度不相同, 体现为具有一定斜率的曲线。
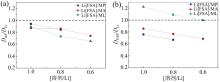 | 图2 30β ℃下不同浓度电解液扩散比值:(a)Dsol/DLi; (b)DFSA/DLi(修改自文献[39])Fig. 2 Diffusivity ratios of different concentrated electrolytes at 30 ° C: (a) Dsol/DLi; (b) DFSA/DLi (modified from reference [39]) |
${{{D}_{\text{sol}}}}/{{{D}_{\text{Li}}}}\; =\frac{a\times [溶剂]}{b\times [\text{Li}]}$ (1)
式中:a、b为系数。
根据式(1), 当 [溶剂]/[Li] = 1.0时, a/b = 0.9为定值, 假设a/b不变, 如果[溶剂]/[Li] = 0.8时, 锂离子浓度相对溶剂浓度是增加, 可得Dsol/DLi = 0.72, 如果数值小于0.72表明DLi变大, 锂离子扩散系数相对溶剂扩散系数是变大的, 在图上表现为在斜率为a/b直线的下方; 如果数值大于0.72表明DLi变小, 锂离子扩散系数相对溶剂扩散系数是减少的。在图上表现为点在斜率为a/b直线的下方。
MP和ML两种电解液随着锂离子浓度的增加, 锂离子自扩散系数相对溶剂扩散系数增加, MA电解液在[溶剂/Li]的比值为1.0和0.8时近似为一条水平直线, 表明锂离子扩散系数相对溶剂扩散系数是减少。当电解液中[溶剂/Li]比值调整为0.6时, 所在点明显在延长线下方, 表明MA电解液中锂离子自扩散系数提高程度在高浓度电解液中更明显。所有电解液随锂离子浓度的增加, 自扩散系数相对阴离子自扩散系数均显著提高。表明提高电解液浓度能够显著提高锂离子的自扩散系数, 进而改善快充电池的性能。
离子在移动过程中会产生离子电流, 某一离子电流占整个溶液离子电流的分数被称为该离子的迁移数[40, 41]。电池在充放电过程中, 锂离子在电解液中将会产生浓度梯度, 浓度梯度的出现将导致离子迁移数出现差异。随着充电电流的增加差异将不断变大。LIU等[42]对提高电池快充性能的电解液进行了研究, 指出电解液的离子迁移数对电池充电倍率起到决定性的作用。如图3(a)所示, 当电池处于充电状态(state of charge, SOC)时, 在正极靠近箔材位置具有最高的锂离子浓度, 随着与正极箔材距离越来越远锂离子浓度逐渐减小, 且在正极和负极中浓度下降并非线性关系。锂离子的浓度变化与正负极材料的孔隙率和曲折度均有关。在隔膜中的锂离子浓度可近似为线性关系。锂离子电池在低SOC下能够以更高的倍率进行充电, 随着SOC状态升高, 允许的最大充电电流逐渐减小, 如图3(b)所示。当电解液的锂离子迁移数变大时, 在同样的SOC状态下电池可承受更高的充电倍率。图3(b)显示, 离子迁移数分别为1.0和0.4的电解液在40% SOC状态下, 电池可以分别承受5.8 C和3.6 C的充电电流。当电池在4.0 C倍率下充电时, 迁移数为1.0的电解液电池可以在70% SOC以下的状态充电。而迁移数为0.4的电解液电池仅满足在20% SOC以下状态充电。综上, 提高电解液的锂离子迁移数可以有效提高电池的快充性能。
 | 图3 (a)锂离子在电池中的浓度梯度示意图; (b)不同离子迁移数下的充电倍率与SOC关系曲线(修改自文献[42])Fig. 3 (a) Concentration gradient diagram of Li+ in a battery; (b) SOC versus charge rate diagram under different ion transport number (modified from reference [42]) |
WU等[43]研究了不同电解液溶剂对锂离子电池快充性能的影响。以1.2 mol/L六氟磷酸锂(LiPF6)为溶质, 以EMC、EC为主溶剂, 加入不同种类的其他溶剂作为共溶剂, 考察不同溶剂配方对电芯快充性能的影响。这些共溶剂包括DMC、MA、EA和EF。溶剂体积配比为EC∶ EMC∶ 共溶剂 = 30∶ 50∶ 20。在20、30、40β ℃温度条件下, 不同溶剂配方电解液的离子导电性大小顺序为MA > EF > EA > DMC, 如图4所示。相关分析认为是由于溶液黏度变化导致电解液性能的不同, 低黏度溶剂能够有效降低电解液中离子运动引起的扩散阻抗。
 | 图4 五种电解液在不同温度下的离子导电性:(a)20β ℃; (b)30β ℃; (c)40β ℃(修改自文献[43])Fig. 4 The ionic conductivities of 5 electrolytes at different temperatures: (a) 20 ° C; (b) 30 ° C; (c) 40 ° C (modified from reference [43]) |
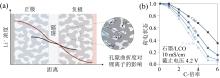
为测试不同配方电解液对快充电池性能的影响, WU等[43]制备LiNi0.6Mn0.2Co0.2O2(NMC622)/石墨软包锂离子电池, 电池容量为520 mA∙ h, 经过化成定容等制程后成为待测软包电池。图5(a)是软包电池在2 C、3 C、4 C倍率下充电后放电容量相对于1 C充电倍率下放电的容量保持率柱状图。在4 C倍率充电条件下性能优劣关系为EA > EF > MA > DMC > EMC, 相关结果差异是由于这些混合溶剂的离子导电性不同引起。高导电性溶剂能够缓解锂离子浓差极化, 保持锂离子浓度的相对均匀性。对注入5种不同电解液电池进行长期循环稳定性测试, 图5(b)表明DMC体系电解液电池具有更优的长期循环稳定性和更高的放电容量。EF体系电解液电池容量快速衰减, EA体系电解液电池容量衰减也比较明显, 虽然EMC体系电解液电池容量衰减慢, 但电池容量发挥却低。对电池进行拆解分析查验负极界面状况, 如图5(b)照片所示。DMC电解液体系的负极界面显示均匀光滑的表面, 但是其他电解液体系的负极界面缺陷比较明显, 特别是EF电解液体系。因此合理的溶剂配方能够抑制电池负极析锂程度, 进而有效提高电池的快充性能。
 | 图5 (a)不同溶剂在不同充电倍率下放电容量保持率; (b)不同溶剂软包电池在快充条件下长期循环性能图及负极石墨电极析锂图(修改自文献[43])Fig. 5 (a) Discharge capacity retention diagrams of different solvents under different rates; (b) long-term cycling performance of the pouch cell under fast charging conditions with different electrolytes and Li plating diagrams of graphite electrodes (modified from reference [43]) |
如图1所示, 电解液中锂离子并非以单个锂离子存在而是与溶剂形成溶剂化锂离子。在锂离子通过SEI膜之前, 溶剂化锂离子需要得到一定的能量来克服势垒而使其去溶剂化最终形成单一的锂离子, 这个能量被称为能垒, 在所有需要克服的能垒中去溶剂化能垒所占比例最大。因此, 低去溶剂化能垒电解液在快充电池中将显示优良的性能[44, 45, 46]。
LEI等[47]研究了EC/DEC、乙腈(acetonitrile, AN)/高浓度电解液(highly concentrated electrolyte, HCE)和AN/稀释高浓度电解液(diluted highly concentrated electrolyte, DHCE)中锂离子与溶剂分子的结合能关系, 从而间接表明何种溶剂需要低的能量来拆散锂离子与溶剂。图6(a)为锂离子在EC/DMC电解液体系、HCE和DHCE电解液体系中的溶剂化结构示意图。在EC/DMC体系电解液中每个锂离子周围被4个EC分子包裹形成溶剂化锂离子。而在HCE和DHCE电解液体系中每个锂离子周围分别被2.28和2.19个AN分子包裹, 这些溶剂化的分子式分别为Li+(EC)4和Li+(AN)2(FSI-)。这些溶剂化锂离子的结合能如图6(b)所示。溶剂化Li+-EC具有最高的结合能, 因此该电解液具有最大的去溶剂化能垒。而溶剂化的Li+-AN具有最低的结合能, 仅需要较低的能量即可实现去溶剂化过程。因此溶剂化结构为Li+-AN和Li+-FSI的电解液能够改善电池的快充性能。
 | 图6 (a)EC/DMC体系电解液中Li+-溶剂结构图; (b)AN/HCE和AN/DHCE体系电解液中Li+-溶剂结构图; (c)三种电解液中溶剂化Li+的结合能(修改自文献[47])Fig. 6 (a) The typical Li+-solvation structure in the EC/DMC system; (b) the typical Li+-solvation structure in the AN-HCE and AN-DHCE systems; (c) the binding energies between Li+and solvents in three different electrolytes (modified from reference [47]) |
图7(a)是锂石墨材料的倍率性能图, 材料在1 C倍率下的容量为350 mA∙ h/g, 在5 C和8 C倍率下的容量分别为325 mA∙ h/g和275 mA∙ h/g, 容量保持率分别为92.8 %和78.6 %, 显示出优良的倍率性能。所制备的软包电池如图7(b)所示。软包电池的倍率性能优良, 在5 C倍率下充电的软包电池最终容量保持率为83 %, 表明电池具有快速充电的能力。软包电池在5 C倍率下进行快速充电, 经过500次循环后容量保持率仍在80%以上, 进一步说明选用的电解液具有快速充电的特点。因此降低电解液的去溶剂化势垒能够明显改善电池的快充能力。
 | 图7 (a)锂石墨材料倍率性能; (b)NCM811/石墨软包电池照片图; (c)NCM811/石墨软包电池倍率性能; (d)NCM811/石墨软包电池5 C倍率下的容量保持率 (修改自文献[47])Fig. 7 (a) Rate performance for lithiation of graphite; (b) optical picture of pouch NCM811/graphite cell; (c) rate performance of pouch NCM811/graphite cell; (d) capacity retention of pouch NCM811/graphite cell at the rate of 5 C (modified from reference [47]) |
ZOU等[48]研究了两种电解液中锂离子与不同溶剂分子之间的结合能随时间变化趋势及溶剂化分子示意图, 如图8所示。
 | 图8 (a)两种电解液中锂离子与溶剂分子结合能图; (b)商用DEC电解液中锂离子去溶剂化示意图; (c)FEC电解液中锂离子去溶剂化示意图(修改自文献[48])Fig. 8 (a) Binding energy of Li+ with two solvents in the electrolytes; (b) schematic diagram of the desolvation process of Li+ in the commercial electrolyte; (c) schematic diagram of the desolvation process of Li+ in the FCE electrolyte (modified from reference [48]) |
图8(a)显示Li+-DEC溶剂化分子结合能为 -20.7 kJ/mol, 而Li+-氟代丙二酸二乙酯(diethyl fluoromalonate, DEFM)溶剂化分子结合能为 -67.5 kJ/mol。经过计算可知, Li+-DEFM的结合能换算为 -0.21 eV, 小于Li+-DEC溶剂化分子结合能。Li+-DEFM溶剂化的电解液具有更弱的溶剂化结构, 有利于锂离子与溶剂分子的拆分。商用电解液中Li+倾向于和DEC溶剂分子配位形成Li+-DEC溶剂壳层, 如图8(b)所示, 配位对结构紧密结合而难于发生去溶剂化过程。相反, 在Li+-DEFM电解液中锂离子与TFSI-是以离子对结构结合, 离子对结构发生去溶剂化过程需要的能量要少于配位对结构。
为了验证电解液对电池性能的影响, 作者测试了两种电解液电池在低温0β ℃下的循环性能, 结果如图9(a)。Li+-DEFM电解液电池在整个循环过程中均表现出稳定的性能, 未出现容量保持率的显著波动, 电池的首次容量为112.1 mA∙ h/g。而采用商用电解液的电池容量下降明显, 首次容量在99 mA∙ h/g左右, 如图9(b)所示。因此, 改变电解液溶剂成分能够有效降低去溶剂化势垒, 提高电池的快充和低温性能。
 | 图9 (a)0β ℃下不同电解液电池循环性能; (b)0β ℃下不同电解液全电池首次充放电曲线(修改自文献[48])Fig. 9 (a) Cycling performances in different electrolytes at 0 ° C; (b) the initial charge-discharge curves of full cells using different electrolytes at 0 ° C (modified from reference [48]) |
在选用相同的溶剂条件下, 不同溶质锂盐对电池的性能也有重要的影响作用[49, 50, 51]。DU等[52]研究了LiPF6和LiFSI溶质锂盐对电池快充性能的影响, 如图10所示。图10(a)表明, 在20 ~ 40β ℃温度范围, 相比于常规LiPF6而言, LiFSI均显示出更高的导电性。不同溶质锂盐具有不同的离子导电性, 因此合适的溶质锂盐能够提高电池的快充性能。制约快充电池性能的另一个因素是电池在充电过程中极化变大, 表现为电池在充电过程中恒流充电较快结束, 电池充电容量大部分是在恒压阶段获得。进一步分析两种不同溶质锂盐电解液在3 C和5 C倍率下快速充电时两种电解液电池恒流充电差异, 如图10(b)。LiFSI溶质锂盐电解液显示出更低的电池极化, 在两种倍率下LiFSI溶质锂盐电解液电池恒流占比均明显大于LiPF6溶质锂盐电解液电池。因此, LiFSI溶质锂盐能够有效提高电池的快充性能。
 | 图10 (a)LiFSI和LiPF6电解液在EC∶ DEC溶剂中的导电性随浓度和温度变化图; 3 C(b)和5 C(c)下电池充电时电压、电流与充电时间关系曲线(修改自文献[52])Fig. 10 (a) Conductivity of LiFSI and LiPF6 in EC:EMC as function of concentration and temperature; voltage and current versus charging time for cells charged at 3 C (b) and 5 C (c) (modified from reference [52]) |
图11是两种电解液电池在经过500次快充循环后电池容量保持, 在整个循环过程中LiFSI溶质锂盐电解液电池容量均高于LiPF6溶质锂盐电解液电池, 随着循环次数的增加两者差异显著增加, 原因是LiPF6溶质锂盐电解液电池在循环过程中负极表面形成更多不可逆的锂金属, 如插图所示。因此, LiFSI溶质锂盐电解液能够降低电池的极化程度, 降低电池快充过程中在负极侧发生析锂的风险。
 | 图11 LiFSI和LiPF6电解液电池在12 min下快充长期循环图(插图为电池循环后负极析锂程度图)(修改自文献[52])Fig. 11 Long term cycling performance of the cells with LiFSI and LiPF6 electrolytes with 12 minutes fast charging (inserted photos show the extent of Li plating on each graphite electrode) (modified from reference [52]) |
在不同体系电池的设计和开发过程中, 电解液在改善电池的性能方面均居于主要地位。以快充电池为例, 改善电池的快充性能需要电池具备薄而致密的SEI膜。致密的SEI膜能够有效保护负极表面, 免于电解液进一步被还原。薄的SEI膜可实现锂离子的快速通过, 进而降低极化并提高电池快充性能。目前快充电池SEI膜的调控需要从溶剂的选择和电解液添加剂的加入等方面开展研究工作。科研人员提出了优化电解液来改善电芯循环性和安全性的方法, 如电解液溶剂的优化选择、溶质盐浓度的改变、电解液添加剂的加入等[53, 54, 55, 56]。
BAEK等[57]报道了采用γ -射线驱使下通过光化学作用形成适合快充电池的人造SEI膜。指出在光的驱使下, 化合物更容易转化为自由基, 卤素元素形成自由基的特点, 使得人造SEI膜中具有更多的无机盐LiF成分。LiF能够稳定负极表面免于被破坏, 而且能够有利于锂离子的快速通过[58, 59]。研究结果显示, 电化学方法形成的SEI膜中成分为LiPF6、LiPxFy、LiF、RCO3-Li和C-O-C五种, 其中前三种无机成分占比为50%左右。而光化学方法形成的SEI膜中成分为LiF、Li2SO4、C-F、RCO3-Li和C-O-C五种, 其中前三种无机成分占比为80%左右, 远高于电化学形成的SEI膜, 且LiF成分的占比为30%左右。
dQ/dV结果显示光化学方法形成的负极还原峰发生明显的右移, 表明光化学方法可以有效改善负极的极化程度, 电池能够更好地适应在大倍率条件下的充电, 如图12(a)所示。图12(b)是两种负极组装成半电池后在不同倍率下的循环结果, 在0.2 C倍率下充电, 两种负极具有相似的容量发挥。当电池以0.5 ~ 2 C倍率充电时, 光化学方法处理的负极显示出优良的倍率性能。可见, 无机成分含量高的SEI膜更有利于电池快充, 因此调控SEI成分及比例是改善电池快充性能的有效方法之一。
 | 图12 (a)石墨负极和光化学石墨负极半电池在0.1 C(35 mA/g)下的dQ/dV曲线图; (b)石墨负极和光化学石墨负极半电池在同倍率下的循环结果(修改自文献[59])Fig. 12 (a) dQ/dV profiles of bare graphite and photo-graphite half-cells during the initial cycles at 0.1 C (35 mA/g); (b) rate capability results of bare graphite and photo-graphite half-cells (modified from reference [59]) |
XU等[60]报道了无机成分和有机成分SEI对锂离子动力学性能的影响, 并采用阿伦尼乌斯方程推算出不同类型SEI的活化能, 如图13所示。图13(a)显示, 对于具有更多无机成分的SEI膜, 锂离子需要消耗较少的能量即可通过, 因此电池具有更佳的抗极化能力。而锂离子通过有机成分占比更多的SEI膜时所需的能量是通过无机占比较多SEI膜的2倍以上。因此, 在选择电解液时需要选取能够形成具有更多无机成分SEI的电解液来提高电池的快充能力。将LiFSI和LiTFSI溶质溶解到DME溶剂中形成4 mol/L LiFSI/DME、1 mol/L LiFSI/DME、1 mol/L LiTFSI/DME电解液。这三种电解液最终形成SEI膜中无机成分占比分别为60.4%(HI-SEI)、37.5%(MI-SEI)和20.2%(LI-SEI)。锂离子通过具有高无机占比的SEI膜所需的能量为62.8 kJ/mol, 显示出强的离子通过能力。锂离子通过具有低无机占比的SEI膜所需的能量为82.7 kJ/mol, 明显要高很多。这种能量差异来源于无机成分能够使得SEI中具有更多适合锂离子通过的路径[61, 62, 63], 如图13(b)中插图所示。
 | 图13 (a)Li+通过富含有机成分和无机成分的SEI时的Ea示意图; (b)离子通过不同SEI时的活化能比较图(修改自文献[60])Fig. 13 (a) Ea schematic illustration of organic-rich and inorganic-rich SEI in the interfacial journey of Li+; (b) comparisons of activation energy of ion transport across different SEI (modified from reference [60]) |
电解液添加剂的加入能够显著改善正负极表面膜性能, 进而提高电池的整体性能[64, 65, 66, 67]。许多科研工作者研究了不同类型电解液添加剂, 这些添加剂的加入均提高了电池的快充性能[68, 69, 70, 71]。YANG等[72]向传统电解液中加入VC和十八烷基铵(octadecylamine, ODA)来改善电池的快充性能。选取LiPF6作为溶质、EC + DMC(EC和DMC体积比为30∶ 70)为溶剂构成基础电解液, 电解质的浓度为1.2 mol/L, 作为无添加剂电解液; 另外, 以分别添加VC、ODA和VC + ODA的电解液作为对照。对注入4种不同电解液的半电池进行阻抗测试, 并进行拟合确定每种电解液下的电荷转移阻抗Rct, 如图14(a)所示, 其中插图为阻抗拟合电路。结果显示在基础电解液中加入VC和ODA均能降低Rct, 两种添加剂对电池的改善程度十分接近, 分别降为基础电解液Rct的66.3 %和65.8 %。将VC和ODA同时添加到基础电解液中后, 电池的Rct改变程度更为明显, 仅为基础电解液Rct的26.2%。阻抗降低的原因是电解液添加剂的加入能够形成更加稳定的SEI膜。VC的加入尽管可以改善SEI膜, 但是成膜不均匀且较粗糙, 而加入ODA的负极形成的SEI膜存在局部破裂的问题。双添加剂的加入使得SEI膜更加均匀且未出现裂纹。为了验证电解液添加剂对电池倍率性能的影响, 对电池分别以0.1、0.5、1、5、10、20 A/g的电流密度进行测试, 结果见图14(b)。双添加剂电解液电池在整个倍率条件下均显示最佳的性能, 在5 A/g电流密度下可发挥800 mA∙ h/g的容量, 远高于其他3种电解液电池。可见合适的电解液添加剂能够显著改善电池的快充性能。
 | 图14 (a)不同添加剂电解液电池拟合后Rct值; (b)注入不同添加剂电解液半电池Li||Ti-SiOx@C在不同倍率下的性能(修改自文献[72])Fig. 14 (a) Rct value with different additive; (b) rate capability of the Li||Ti-SiOx@C half cells in the different electrolytes under various current densities (modified from reference [72]) |
为了验证电解液是否适合高镍三元正极与硅碳负极构成的全电池体系, 将同批次制备的电池分别注入上述4种电解液, 测试结果如图15。经过1 000次循环后, ODA添加剂电解液电池仅具有10.8%的初始容量保持, 不如基础电解液电池14.7%的保持率。VC添加剂加入可以有效提升电池的快充性能。而双添加剂电解液电池既可以克服VC添加剂电池负极SEI膜不均匀的问题也可以克服ODA添加剂负极SEI膜局部出现裂纹的问题, 使得负极SEI膜具有薄而均匀的特点。
 | 图15 在NCM-811||Ti-SiOx@C电池体系下不同电解液以5 C倍率进行长循环的曲线图(修改自文献[72])Fig. 15 Cycling performance of the NCM-811||Ti-SiOx@C full cells in the different electrolytes at 5 C (modified from reference [72]) |
尽管新能源电动汽车续航里程在不断增加, 里程焦虑问题在一定程度上得到有效缓解, 然而如何缩短电动汽车充电时间成为吸引消费者购买的重要制约因素。开发出能够实现在10 ~ 15 min内充满电的快充电池成为实现电动汽车全面普及的有效保障。如何设计开发出具有快充特点的电池需要从正极材料、负极材料和电解液的选择上展开。对于快充电池, 可以把电解液形象地比喻为电池的血液, 血液质量的好坏将直接决定整个电池的寿命。因此, 电解液开发和创新是电池性能提高的关键要素。当然, 快充电池的开发也要充分考虑电池在高倍率条件下充电时的产热, 以及当前充电桩是否满足实际电池包的充电要求。因此, 电池包系统应具有在低温环境下的加热功能及在高温条件下的制冷功能, 以实现电池包在全气候条件下的工作能力。
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|


