0 引言
1 计算模型
1.1 井筒内CaCO3结垢基本物理化学机理
1.2 计算模型建立
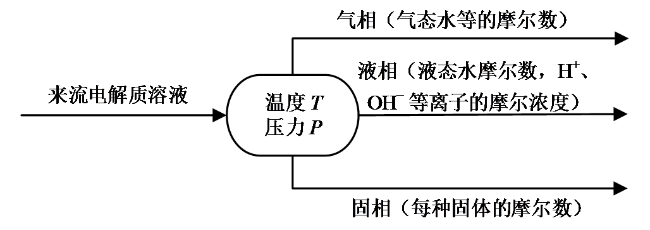
Fig. 1 Schematic of phase equilibrium model of electrolyte solution图1 电解质溶液相平衡模型图 |
1.3 模型求解算法
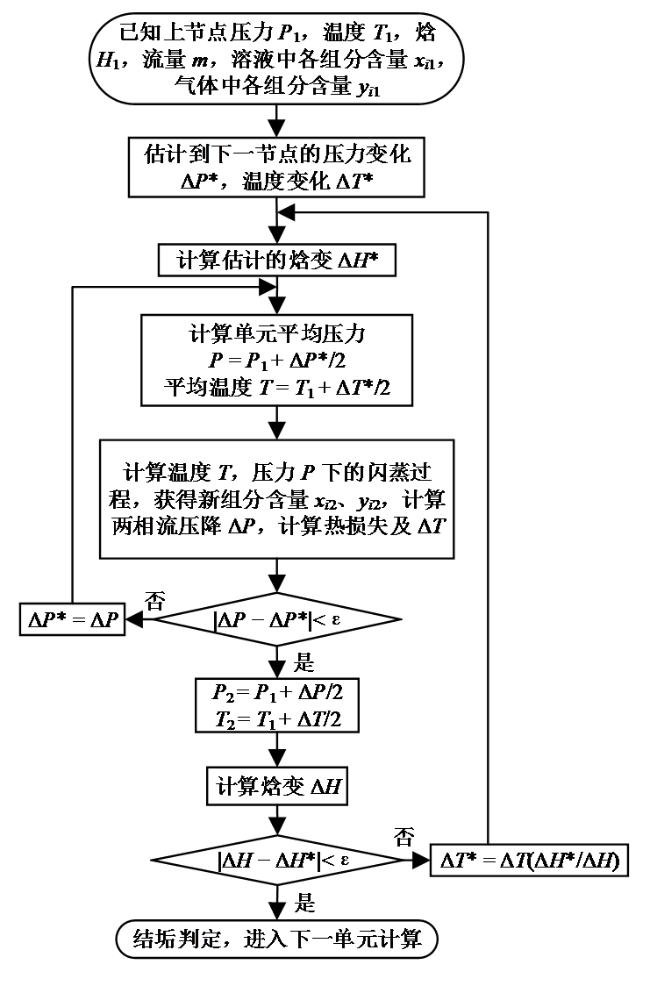
Fig. 2 Flow chart of a calculation unit图2 单元计算流程图 |
1.4 化学反应方程和相变平衡方程
Table 1 Chemical reaction and phase change equilibrium equations involved in this simulation program表1 计算程序所涉及的化学反应方程和相变平衡 |
| 序号 | 方程式 |
|---|---|
| 1 | ${{\text{H}}_{2}}\text{O}+\text{C}{{\text{O}}_{2(\text{aq})}}\leftrightarrow \text{HC}{{\text{O}}_{3}}^{-}+{{\text{H}}^{+}}$ |
| 2 | $\text{HC}{{\text{O}}_{3}}^{-}\leftrightarrow \text{C}{{\text{O}}_{3}}^{2-}+{{\text{H}}^{+}}$ |
| 3 | ${{\text{H}}_{\text{2}}}\text{O}\leftrightarrow {{\text{H}}^{+}}+\text{O}{{\text{H}}^{-}}$ |
| 4 | $\text{CaHC}{{\text{O}}_{3}}^{+}\leftrightarrow \text{C}{{\text{a}}^{\text{2+}}}+\text{HC}{{\text{O}}_{3}}^{-}$ |
| 5 | $\text{CaC}{{\text{O}}_{3}}^{0}\leftrightarrow \text{C}{{\text{a}}^{2+}}+\text{C}{{\text{O}}_{3}}^{2-}$ |
| 6 | $\text{Ca(OH}{{\text{)}}^{+}}\leftrightarrow \text{C}{{\text{a}}^{2+}}+\text{O}{{\text{H}}^{-}}$ |
| 7 | ${{\text{H}}_{2}}{{\text{O}}_{\text{(aq)}}}\leftrightarrow {{\text{H}}_{2}}{{\text{O}}^{\text{g}}}$ |
| 8 | $\text{C}{{\text{O}}_{2(\text{aq})}}\leftrightarrow \text{C}{{\text{O}}_{2}}^{\text{g}}$ |
| 9 | $\text{NaC}{{\text{l}}^{\text{s}}}(\text{岩盐)}\leftrightarrow \text{N}{{\text{a}}^{+}}+\text{C}{{\text{l}}^{-}}$ |
| 10 | $\text{CaC}{{\text{O}}_{3}}^{\text{s}}(\text{方解石)}\leftrightarrow \text{C}{{\text{a}}^{2+}}+\text{C}{{\text{O}}_{3}}^{2-}$ |
| 11 | $\text{C}{{\text{H}}_{4(\text{aq)}}}\leftrightarrow \text{C}{{\text{H}}_{4}}^{\text{g}}$ |
注:下角标aq表示在水溶液中,g表示气相,s表示固相。 |
2 结果与分析
2.1 算例分析
Table 2 Concentration of each component in the geothermal water at different depths of the well表2 井内地热水不同深度下各组分浓度 |
| 条件 | 物质浓度 / (mol/kgw) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H+ | OH- | HCO3- | CO32- | CO2(aq) | CH4(aq) | Ca2+ | CaHCO3+ | CaCO30 | Ca(OH)+ | H2O(aq) | H2O(g) | CO2(g) | CH4(g) | |
| h = 1 000 m T = 343.15 K P = 112.0 bar | 2.84×10-6 | 5.90×10-8 | 2.02×10-4 | 5.47×10-9 | 1.13×10-3 | 1.25×10-2 | 9.93×10-5 | 4.81×10-7 | 3.52×10-9 | 2.62×10-7 | 55.540 76 | 0.00 | 0.00 | 0.00 |
| h = 150 m T = 342.436 K P = 18.7 bar | 2.81×10-6 | 5.53×10-8 | 2.02×10-4 | 5.44×10-9 | 1.13×10-3 | 1.25×10-2 | 9.93×10-5 | 4.79×10-7 | 3.42×10-9 | 2.32×10-7 | 55.540 76 | 0.00 | 0.00 | 0.00 |
| h = 60 m T = 342.278 K P = 8.8 bar | 8.51×10-6 | 1.74×10-8 | 6.81×10-5 | 6.08×10-10 | 1.16×10-3 | 7.28×10-3 | 2.99×10-5 | 4.86×10-8 | 1.15×10-10 | 2.27×10-8 | 55.540 81 | 1.82×10-5 | 4.33×10-5 | 5.22×10-3 |
| h = 0 m T = 342.164 K P = 2.2 bar | 6.81×10-6 | 2.15×10-8 | 6.64×10-5 | 7.40×10-10 | 9.00×10-4 | 1.68×10-3 | 2.99×10-5 | 4.74×10-8 | 1.39×10-10 | 2.80×10-8 | 55.540 67 | 1.6×10-4 | 3.00×10-4 | 1.08×10-2 |
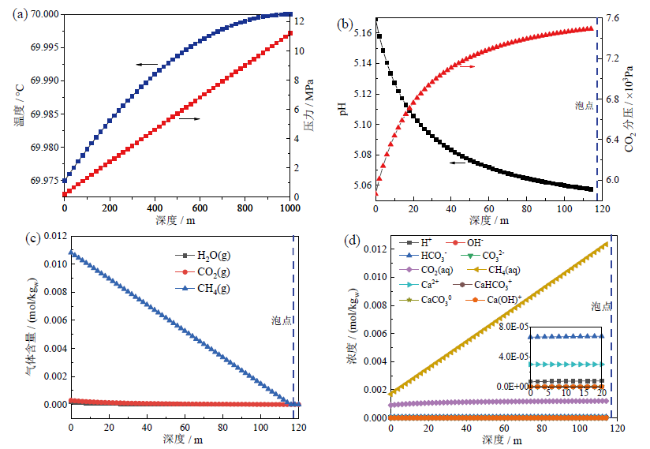
Fig. 3 (a) Temperature and pressure distribution along the well; (b) CO2 partial pressure and pH evolution after bubble point; (c) concentration evolution of gas components after bubble point; (d) concentration evolution of liquid components after bubble point图3 (a)温度压力随井深变化曲线;(b)泡点之后CO2分压和pH值随井深变化曲线;(c)泡点之后气相组成随井深变化曲线;(d)泡点后溶液中各组分变化情况 |
2.2 不凝性气体含量对结垢量和泡点位置的影响分析
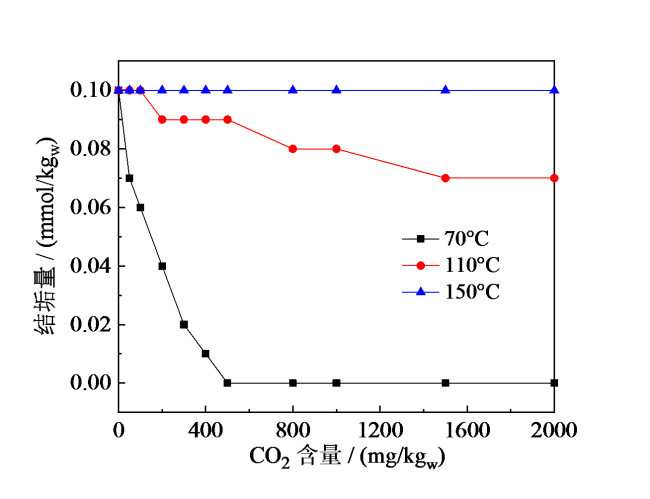
Fig. 4 Relation of scale quantities with CO2 concentration under different well fluid temperatures图4 不同温度下结垢量与CO2含量关系 |
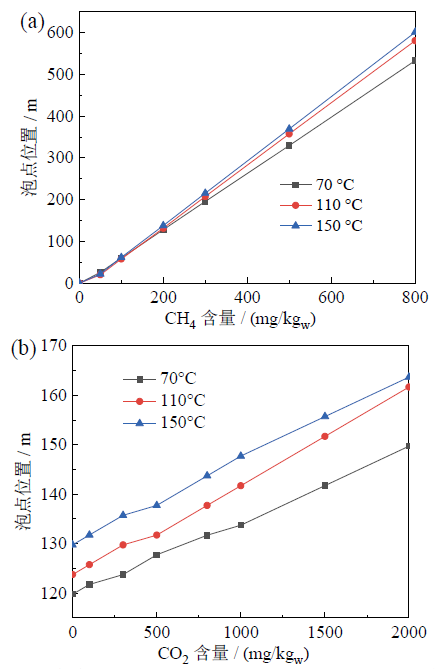
Fig. 5 The effect of CH4 (a) and CO2 (b) concentration on bubble point图5 地热水中CH4含量(a)和CO2含量(b)对泡点位置的影响 |